Mammalian protein found in Homo sapiens
| CYGB |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1UMO, 1URV, 1URY, 1UT0, 1UX9, 1V5H, 2DC3, 3AG0, 4B3W |
|
|
| Identifiers |
|---|
| Aliases | CYGB, HGB, STAP, cytoglobin |
|---|
| External IDs | OMIM: 608759; MGI: 2149481; HomoloGene: 12706; GeneCards: CYGB; OMA:CYGB - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 17 (human)[1] |
|---|
| | Band | 17q25.1 | Start | 76,527,356 bp[1] |
|---|
| End | 76,551,175 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 11 (mouse)[2] |
|---|
| | Band | 11|11 E2 | Start | 116,536,421 bp[2] |
|---|
| End | 116,545,139 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - subcutaneous adipose tissue
- right lobe of thyroid gland
- left lobe of thyroid gland
- gastric mucosa
- canal of the cervix
- tibial nerve
- urinary bladder
- right coronary artery
- left coronary artery
- left uterine tube
|
| | Top expressed in | - interventricular septum
- aortic valve
- ascending aorta
- habenula
- dorsal tegmental nucleus
- ankle
- lateral hypothalamus
- medial vestibular nucleus
- superior colliculus
- cardiac muscles
|
| | More reference expression data |
|
|---|
| BioGPS | 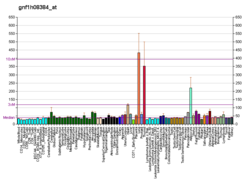 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - fatty acid peroxidase activity
- metal ion binding
- heme binding
- peroxidase activity
- catalase activity
- nitric oxide dioxygenase activity
- oxygen binding
- oxygen carrier activity
- iron ion binding
- protein binding
| | Cellular component | - cytoplasm
- cytosol
- neuron projection
- neuronal cell body
- nuclear speck
| | Biological process | - response to hypoxia
- response to oxidative stress
- negative regulation of hepatic stellate cell activation
- negative regulation of collagen biosynthetic process
- fatty acid oxidation
- regulation of nitric-oxide synthase activity
- negative regulation of fibroblast migration
- cellular oxidant detoxification
- oxygen transport
| | Sources:Amigo / QuickGO |
|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Cytoglobin is the protein product of CYGB, a human and mammalian gene.[5]
Cytoglobin is a globin molecule ubiquitously expressed in all tissues and most notably utilized in marine mammals. It was discovered in 2001[6] and named cytoglobin in 2002.[7] It is thought to protect against hypoxia. The predicted function of cytoglobin is the transfer of oxygen from arterial blood to the brain.[8]
Function
Cytoglobin is a ubiquitously expressed hexacoordinate hemoglobin that may facilitate diffusion of oxygen through tissues, scavenge nitric oxide or reactive oxygen species, or serve a protective function during oxidative stress.[5][9]
Applications
CYGB expression can be used as a specific marker with which hepatic stellate cells can be distinguished from portal myofibroblasts in the damaged human liver.[10]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000161544 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020810 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: CYGB cytoglobin".
- ^ Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K (Jul 2001). "Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells". The Journal of Biological Chemistry. 276 (27): 25318–23. doi:10.1074/jbc.M102630200. PMID 11320098.
- ^ Burmester T, Ebner B, Weich B, Hankeln T (Apr 2002). "Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues". Molecular Biology and Evolution. 19 (4): 416–21. doi:10.1093/oxfordjournals.molbev.a004096. PMID 11919282.
- ^ "Why Diving Marine Mammals Resist Brain Damage from Low Oxygen". ScienceDaily. 20 December 2007.
- ^ Trent JT, Hargrove MS (May 2002). "A ubiquitously expressed human hexacoordinate hemoglobin". The Journal of Biological Chemistry. 277 (22): 19538–45. doi:10.1074/jbc.M201934200. PMID 11893755.
- ^ Motoyama H, Komiya T, Thuy le TT, Tamori A, Enomoto M, Morikawa H, Iwai S, Uchida-Kobayashi S, Fujii H, Hagihara A, Kawamura E, Murakami Y, Yoshizato K, Kawada N (Feb 2014). "Cytoglobin is expressed in hepatic stellate cells, but not in myofibroblasts, in normal and fibrotic human liver". Laboratory Investigation. 94 (2): 192–207. doi:10.1038/labinvest.2013.135. PMID 24296877.
Further reading
- Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K (Jul 2001). "Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells". The Journal of Biological Chemistry. 276 (27): 25318–23. doi:10.1074/jbc.M102630200. PMID 11320098.
- Burmester T, Ebner B, Weich B, Hankeln T (Apr 2002). "Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues". Molecular Biology and Evolution. 19 (4): 416–21. doi:10.1093/oxfordjournals.molbev.a004096. PMID 11919282.
- Asahina K, Kawada N, Kristensen DB, Nakatani K, Seki S, Shiokawa M, Tateno C, Obara M, Yoshizato K (Sep 2002). "Characterization of human stellate cell activation-associated protein and its expression in human liver". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1577 (3): 471–5. doi:10.1016/s0167-4781(02)00477-3. PMID 12359339.
- Sawai H, Kawada N, Yoshizato K, Nakajima H, Aono S, Shiro Y (May 2003). "Characterization of the heme environmental structure of cytoglobin, a fourth globin in humans". Biochemistry. 42 (17): 5133–42. doi:10.1021/bi027067e. PMID 12718557.
- Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L (Aug 2003). "A globin in the nucleus!". The Journal of Biological Chemistry. 278 (33): 30417–20. doi:10.1074/jbc.C300203200. PMID 12796507.
- Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC (Dec 2003). "The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin". The Journal of Biological Chemistry. 278 (51): 51713–21. doi:10.1074/jbc.M309396200. PMID 14530264.
- Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, Simpson JC, Nevo E, Saaler-Reinhardt S, Reuss S, Hankeln T, Burmester T (Feb 2004). "Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia". The Journal of Biological Chemistry. 279 (9): 8063–9. doi:10.1074/jbc.M310540200. PMID 14660570.
- Hünermund G, Schirmacher A, Ringelstein B, Young P, Watts GD, Meuleman J, Nelis E, Chance PF, Timmerman V, Stögbauer F, Kuhlenbäumer G (Apr 2004). "Genomic organization and mutation analysis of three candidate genes for hereditary neuralgic amyotrophy". Muscle & Nerve. 29 (4): 601–4. doi:10.1002/mus.20009. PMID 15052627. S2CID 39945876.
- de Sanctis D, Dewilde S, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M (Feb 2004). "Crystal structure of cytoglobin: the fourth globin type discovered in man displays heme hexa-coordination". Journal of Molecular Biology. 336 (4): 917–27. doi:10.1016/j.jmb.2003.12.063. PMID 15095869. S2CID 21144924.
- Sugimoto H, Makino M, Sawai H, Kawada N, Yoshizato K, Shiro Y (Jun 2004). "Structural basis of human cytoglobin for ligand binding". Journal of Molecular Biology. 339 (4): 873–85. doi:10.1016/j.jmb.2004.04.024. PMID 15165856.
- Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE (Oct 2004). "Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance". The Journal of Biological Chemistry. 279 (43): 44417–26. doi:10.1074/jbc.M407126200. PMID 15299006.
- Hamdane D, Kiger L, Dewilde S, Uzan J, Burmester T, Hankeln T, Moens L, Marden MC (Apr 2005). "Hyperthermal stability of neuroglobin and cytoglobin". The FEBS Journal. 272 (8): 2076–84. doi:10.1111/j.1742-4658.2005.04635.x. PMID 15819897. S2CID 13377138.
- Sawai H, Makino M, Mizutani Y, Ohta T, Sugimoto H, Uno T, Kawada N, Yoshizato K, Kitagawa T, Shiro Y (Oct 2005). "Structural characterization of the proximal and distal histidine environment of cytoglobin and neuroglobin". Biochemistry. 44 (40): 13257–65. doi:10.1021/bi050997o. PMID 16201751.
- Shaw RJ, Liloglou T, Rogers SN, Brown JS, Vaughan ED, Lowe D, Field JK, Risk JM (Feb 2006). "Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: quantitative evaluation using pyrosequencing". British Journal of Cancer. 94 (4): 561–8. doi:10.1038/sj.bjc.6602972. PMC 2361183. PMID 16449996.
- McRonald FE, Liloglou T, Xinarianos G, Hill L, Rowbottom L, Langan JE, Ellis A, Shaw JM, Field JK, Risk JM (Apr 2006). "Down-regulation of the cytoglobin gene, located on 17q25, in tylosis with oesophageal cancer (TOC): evidence for trans-allele repression". Human Molecular Genetics. 15 (8): 1271–7. doi:10.1093/hmg/ddl042. PMID 16510494.
- Xinarianos G, McRonald FE, Risk JM, Bowers NL, Nikolaidis G, Field JK, Liloglou T (Jul 2006). "Frequent genetic and epigenetic abnormalities contribute to the deregulation of cytoglobin in non-small cell lung cancer". Human Molecular Genetics. 15 (13): 2038–44. doi:10.1093/hmg/ddl128. PMID 16698880.
External links
PDB gallery
-
1umo: THE CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN -
1urv: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION -
1ury: CYTOGLOBIN CAVITIES -
1ut0: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION -
1ux9: MAPPING PROTEIN MATRIX CAVITIES IN HUMAN CYTOGLOBIN THROUGH XE ATOM BINDING: A CRYSTALLOGRAPHIC INVESTIGATION -
1v5h: Crystal Structure of Human Cytoglobin (Ferric Form) -
2dc3: Crystal structure of human cytoglobin at 1.68 angstroms resolution |
|
|---|
| Globins | |
|---|
| Other | |
|---|
see also disorders of globin and globulin proteins |
 | This article on a gene on human chromosome 17 is a stub. You can help Wikipedia by expanding it. |

 1umo: THE CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN
1umo: THE CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN 1urv: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION
1urv: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION 1ury: CYTOGLOBIN CAVITIES
1ury: CYTOGLOBIN CAVITIES 1ut0: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION
1ut0: CRYSTAL STRUCTURE OF CYTOGLOBIN: THE FOURTH GLOBIN TYPE DISCOVERED IN MAN DISPLAYS HEME HEXA-COORDINATION 1ux9: MAPPING PROTEIN MATRIX CAVITIES IN HUMAN CYTOGLOBIN THROUGH XE ATOM BINDING: A CRYSTALLOGRAPHIC INVESTIGATION
1ux9: MAPPING PROTEIN MATRIX CAVITIES IN HUMAN CYTOGLOBIN THROUGH XE ATOM BINDING: A CRYSTALLOGRAPHIC INVESTIGATION 1v5h: Crystal Structure of Human Cytoglobin (Ferric Form)
1v5h: Crystal Structure of Human Cytoglobin (Ferric Form) 2dc3: Crystal structure of human cytoglobin at 1.68 angstroms resolution
2dc3: Crystal structure of human cytoglobin at 1.68 angstroms resolution